
Vivasure Medical, a medtech company based in Galway, Ireland, has developed the PerQseal device, a synthetic implant designed to seal large bore blood vessel punctures. The implant has utility in a wide variety of transcatheter endovascular procedures, such as transcatheter aortic valve replacement (TAVR), thoracic endovascular aneurysm repair (TEVAR), and endovascular abdominal aneurysm repair (EVAR), and aims to significantly improve on current approaches to close large vessel punctures.
The implant is an intravascular patch that is applied to the puncture from inside the vessel and is fully absorbable. The patch does not require any sutures and aims to reduce the chances that negative issues will develop post procedure, such as bleeding complications.
Vivasure has recently released a new version of the device, called the PerQseal+, that aims to provide a more robust solution in reducing the potential for complications and to enhance control and tactile feedback for the operator. The company recently announced the enrollment of the first patient in the Frontier V trial, a European multicenter study of the PerQseal+ device.
Medgadget had the opportunity to speak with Andrew Glass, CEO of Vivasure Medical, about the technology.
Conn Hastings, Medgadget: Please give us an overview of the types of percutaneous procedures in which large bore vessel punctures are involved.
Andrew Glass, Vivasure Medical: Over the past decade, cardiovascular surgical procedures have transitioned more and more to minimally invasive approaches. Large hole arterial access, usually through the leg, is required for clinicians to perform these percutaneous cardiovascular procedures, including transcatheter aortic valve replacement (TAVR), thoracic and abdominal endovascular aneurysm repair (TEVAR and EVAR) and for the use of a cardiac assist device (CAD).
Medgadget: How are large bore vascular punctures currently sealed? What are the disadvantages with these approaches?
Andrew Glass: The current approach to large diameter arterial closure is a surgical repair or the use of suture- or collagen-based closure devices. Both result in major vascular complications ranging from 5–15%. This has been observed in many publications for TAVR where vascular events from the closure of the arteriotomy are the most common complication of the procedure. The challenge with current techniques is the impact on vessel integrity. Large vessel closure with current techniques is often associated with vessel distortion at the closure site which may lead to stenosis, thrombus formation or abrupt closure.
Medgadget: Please give us an overview of the PerQseal device and how it works.
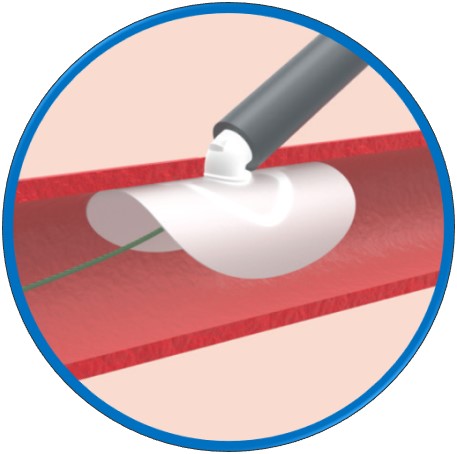
Andrew Glass: PerQseal is the first sutureless, fully absorbable synthetic implant for large-bore arterial punctures. The PerQseal technology consists of an intravascular patch that seals the vessel from the inside, returning the artery to its natural state without any sutures, metal implants or collagen left behind. For other closure devices, up to 30 steps are needed to deploy. We optimized the deployment of our device to 10 total steps, reducing the amount of training required.
Medgadget: How does the system improve on other vessel closure techniques?
Andrew Glass: With current closure techniques, there is a moment during the procedure where the vessel is open and the clinician needs to close quickly to reduce unnecessary bleeding. Because our approach involves a seal being placed from inside the vessel, we believe our deployment is simpler and more controlled than conventional closure techniques. PerQseal does not require time-consuming pre-closure steps, allowing for patient comfort and a reduction in total procedure time. Unlike other closure technologies, the PerQseal is a fully bioabsorbable solution that leaves nothing behind in the vessel. This has numerous potential advantages such as re-access to the vessel and long-term safety for the patient.
Medgadget: How was the device conceived and how did you identify a need for a dedicated closure system for large catheters?
Andrew Glass: While less invasive procedures have been increasingly popular over the past decade, sometimes they can be associated with bleeding complications and other challenges – so safe, rapid and efficient vessel closure is important for patient outcomes and to keep costs associated with adverse events as low as possible. Vivasure, working with an international group of clinicians, identified the need for an effective vascular closure device for large arterial punctures. Looking at pressurized models, it’s logical that sealing from the inside is easier than sealing from the outside. This served as the basis for our approach, and from day one, we sought a fully bioabsorbable solution that is simple to deploy for clinicians and aims to be safer for the patient. This resulted in our PerQseal product.
Medgadget: How does the new PerQseal+ device differ from the first generation device?
Andrew Glass: The enhanced PerQseal+ device is intended to provide clinicians with an even more robust solution for managing challenges and bleeding complications associated with large-bore closure. The device design is optimized to provide enhanced control and tactile feedback for the clinician while providing the user added scope for managing a wider range of vessel wall damage that may occur during large catheter procedures. This is particularly the case in the growing elderly population undergoing cardiovascular procedures where the arteries tend to be more calcified and less resilient, making them more susceptible to complications.
Medgadget: Please give us an overview of the ongoing Frontier V study.
Andrew Glass: The Frontier V study is a multicenter clinical trial in Europe evaluating the next-generation PerQseal+ device with an enhanced bioabsorbable patch designed to address more complex patient anatomies undergoing percutaneous cardiovascular procedures. 25 patients will be enrolled in the first phase of the Frontier V study followed by the second phase with up to 90 patients across Germany and the Netherlands. Enrollment is expected to complete this year and the resulting data will serve as a basis for our U.S. clinical plan.

Link: Vivasure Medical homepage…
Flashbacks: PerQseal Absorbable, Sutureless Large-Bore Closure Device Coming to Europe; Bioabsorbable PerQseal Vivasure Large Bore Femoral Closure Device Cleared in EU;
